On-Demand Outsourcing BPO Services for Healthcare Providers With 24/7 Coverage!
Save up to 70% on staffing costs!
Browse Specialty Staffing ServicesMedical Coding Process

Author: Bhavika Birpan
Data generated in all clinical trials are recorded on data collection instrument case report from Electronic case report from By investigators located at various sites in various countries. In multicentric clinical trials, different investigators or medically qualified experts are from different sites. Center recording the medical terms uniformly is a big challenge. medical coders from the clinical data management team process these teams and perform medical coding is performed to categorize the medical terms reported appropriately so that they can be analyzed and reviewed. this article describes the process which is used for medical coding clinic data management and the two most commonly used medical dictionaries MedDRA and WHO-DDE
Data generated ai all clinical trials are recorded in data collection instruments (DCIs) called case record forms. Report forms (CRFs) in respect of paper-based trials or as electronic Case Record report forms (eCRF) in respect of web-based clinic trials. dications (CM) used in addition to the study drug are collected and recorded on the relevant DCIS. A multicenter clinical trial consists of multiple trial sites, involving different investigators from different ethnic backgrounds. It is anticipated that the participation of investigators and clinical research professionals from different countries/regions is likely to record medical/scientific data differently. All data generated in these tests are ultimately subject to further analysis. It is imperative that this data is uniformly interpreted in a standardized format. Therefore medical coding using standardized medical dictionaries is essential. Data listed above such as AES, SAES, MH, CM, and any other category are usually coded. However, AES, SAES, and CM coding are mandated in any given clinical trial
There are several standardized medical coding dictionaries in the market however five dictionaries listed below are used for coding.
- COSTART – Coding Symbols for Thesaurus of Adverse Reactions Terms
- ICD9CM – International Classification of Diseases 9th Revision Clinical Change
- MedDRA– Medical Dictionary for Regulatory Activities
- WHO-ART– World Health Organization Adverse Reaction Terminology
- WHO-DDE– World Health Organization Drug dictionary enhanced
the above five, the two widely used medical coding dictionaries used to code medical terms arising in clinical trials are MedDRA and WHO-DDE. It is nearly impossible to maintain uniformity in the reporting of a term in any clinical trial. However, it is a challenging task for a coder to ensure that the word recorded/reported on the data collection device (CRF/eCRF) is coded appropriately. It is well-known fact that these dictionaries come at a cost and organizations that undertake medical coding activity must have appropriate valid licenses. Appropriately, separate licenses are issued for each dictionary for different user groups.
Process in Medical Coding
Precoding Process: Any Medical Coding Dictionary and all subsequent modifications have to be correctly imported and loaded into the appropriate coding tool by the database programming team. The coding tool used in connection with Oracle Clinical (OC) is Thesaurus Management System (TMS). Once the dictionaries are imported/loaded into the appropriate tool, the programming team checks whether all the tables/records in the tool are loaded correctly. This process is performed only once for a given version of the dictionary. After the programming team ensures the correct import tables and records into the tool, the operational team conducts a User Acceptance Test (UAT), in which members of the operational team confirm that the dictionary loaded into the tool is producing the expected output as expected. Once the operational team approves the UAT, the selected dictionary is released for use by a particular project study. If the same version of the dictionary is to be used for another project/study in the future, the UAT should be repeated by members of the operational team assigned to the new project study.
Before assigning a dictionary for a project/study, it is necessary to check the following points as pre-requisite.
- The latest valid version of the coding tool available at the start of the project
- Policy requirement regarding the use of the same version of the dictionary available in the tool for the entire project spite the availability of a newer version
- Upgrade version whenever available during the life of the project
- upgraded versions of the project as they become available during the project’s life cycle of the project.
Coding in Project
Coding is done on data that has been validated and cleaned, ideally by data managers responsible for “data review and discrepancy management”. Terms to be coded in any project are coded by a process called “auto coding”, terms that fail to get “auto-coded” have to be coded “manually” by the medical coder responsible for the project. The two processes of coding – auto and manual are briefly described below
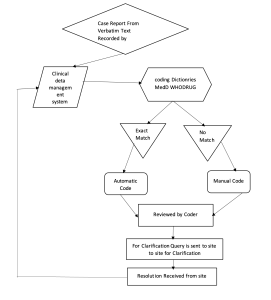
Auto Coding: The word entered by the investigator on the data collection device is automatically coded if it exactly matches the appropriate word available in the medical dictionary.
Manual Coding: Auto coding fails in respect of words that do not match the appropriate level of hierarchy in the medical dictionary. All these conditions needed to be coded manually by the medical coder assigned to the project. The medical coder will find the appropriate match for the word from among the words in the assigned dictionary and manually specify the code.
This does not mean that all terms reported and recorded on CRF / eCRF get coded without any issues. There are some terms that are unclear or for which it is not very easy for a coder to find matching terms within the dictionary. The investigator may report multiple signs and symptoms. In such cases, the medical coder/medical coding team sends these terms to investigators/medically qualified experts for clarification/more information. It helps the medical coder to identify term(s) very close to such unclear or doubtful terms within the coding dictionary so that the term(s) get appropriately coded. Term(s) which get auto and manually coded are reviewed by the coding personnel. Unclear term(s)/term(s) with insufficient details are queried to the site. An investigator must provide appropriate updates/details and send the signed resolution back to the data management team. Based on the investigator’s resolution, the data management team takes appropriate action in the database. The coder looks at the information/update and then codes the term appropriately.
The Same Process is depicted in Flow Chart Below
Medical Dictionary for Regulatory Activities (MedDRA)
Medical Dictionary for Regulatory Activities (MedDRA®) is a medical coding dictionary developed by the Maintenance and Support Services Organization (MSSO). MedDRA® is supported by the International Conference on Harmonization (ICH) on Technical Requirements for the Registration of Pharmaceuticals for Human Use. Prior to the development of MedDRA, there was no internationally accepted medical terminology for biopharmaceutical regulatory purposes.
MedDRA is used for Coding
- Medical terms generated during all phases of the clinical trial, except animal toxicology,
- Therapeutic indications that include signs, symptoms, disease, diagnosis, or prevention of disease, and modification of functions
- Coding names and quantitative results of investigations, surgical procedures, and medical/social/family history.
MedDRA has five hierarchical levels as listed below
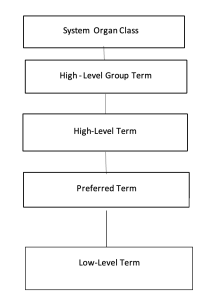
- Low Level Duration (LLT)
- Favorite Temple (PT)
- High Level Duration (HLT)
- High Level Group Temp (HLGT) System Organ Class (SOC)
Low Level Term (LLT) is the lowest level of vocabulary. Each LLT is connected to only one PT. A PT clearly describes a symptom, sign, disease, diagnosis, medical indication, investigation, surgical, or medical procedure and the medical, social, or family history characteristics.
High Level Term (HLT) is a superordinate descriptor for the PT associated with it. High Level Group Term (HLGT) is a superordinate descriptor for one or more HUTS related to anatomy, pathology, physiology, etiology or function. System Organ Class (SOC) is the highest level of the hierarchy. SOC are grouped by etiology, expression site, and purpose
Common problems faced by medical coding specialist while coding
- illegible verbatim term
- Spelling errors
- Use of abbreviations
- Multiple signs and symptoms recorded as separate events that may lead to some diagnosis (for example: symptoms recorded as runny nose, cough, and fever may lead to a diagnosis of pneumonia)
- Multiple medical concepts are recorded together. To code we need to split the terms.
- The incident is recorded without mentioning the site e.g. Ulcers are recorded without additional information like moth ulcer, leg ulcer etc.
- Multiple medical concepts were recorded including the surgical procedure and the cause of the injury. Although not the cause or cause or site of the injury clarified.
- A medication term reported however allergy due to the medication or outcome allergy is not Specified
Other Commonly used dictionaries
World Health Organization Drug Dictionary (WHODRUG): This is a dictionary maintained and updated by the Uppsala Monitoring Center (UMC). This dictionary is the most comprehensive dictionary containing medicinal product information. It is used by drug regulatory authorities, various pharmaceutical companies, and contract research organizations (CROs). The dictionary includes medicinal product names – proprietary and non-proprietary – from over 90 countries. The WHODRUG dictionary has evolved significantly. We currently have three dictionary types
- WHO Drug Dictionary (WHO-DD)
- WHO Drug Dictionary Enhanced (WHO-DD Enhanced)
- WHO Herbal Dictionary (WHO-HD)
The WHO DD and WHO DD Enhanced contain information mainly on traditional medicinal products, but several other product types are listed below:
- Medicinal Products
- Herbal Remedies
- vaccine
- dietary supplement
- Radio-medicine
- Blood Products
- Diagnostic Agent
- Homeopathic remedy
The WHO Herbal Dictionary includes almost all herbal entries that have been recorded in the WHO Drug Dictionary over the years. From 2005 all herbs will be specifically included in the WHO Herbal Dictionary. The WHO Herbal Dictionary classifies herbals with the Herbal Anatomical Therapeutic Chemical (HATC) classification.
The WHO Drug Dictionary contains information on medicinal products. This information is used to identify a term (medicinal product) that closely matches the term reported on DCI.
An integral part of the ATC classification dictionary. It is used to classify the medicinal product for the main therapeutic use of the active ingredient
Level 1: Body Core Groups
Level 2: Therapeutic Subgroup
Level 3: Pharmacological Subgroup
Level 4: Chemical Subgroup
Level 5: Chemicals
The ATC classification for the medicinal pro metformin according to the above system would be as follows:
A Alimentary tract and metabolism
(1st level, anatomical main group)
A10 Drugs used in diabetes
(2nd level, therapeutic subgroup)
A10B Blood glucose lowering drugs, excl. insulins (3rd level, pharmacological subgroup)
A10BA Biguanides
(4th level, chemical subgroup)
A10BA02 Metformin
(5th level, chemical substance)
Common problems faced by the medical coding specialist while coding medicinal products:
- illegible verbatim term
- Spelling errors
- Use of abbreviations
- The prescribed indication for the medicinal product is not an approved indication mentioned on the prescribing information
- Local brand available in the market and generic/active ingredient not known.
- Multiple drugs entered at once. To code we need to split the terms.
 Book a Demo to Build Your Team Today!
Book a Demo to Build Your Team Today!
 Read Case Studies
Read Case Studies 


 Virtual Medical Assistants
Virtual Medical Assistants



